Crystal systems
There are many crystal polyhedrons, and they all differ in their specific degree of symmetry. Studying a crystal, which above all involves measuring the angles between crystal faces, gives us basic ELEMENTS OF SYMMETRY (centre of symmetry, plane of symmetry, axis of symmetry) of that crystal polyhedron.
There are 32 different combinations of elements of symmetry that define the external symmetry of a crystal, and so we can say that all mineral species, and an even larger number of all crystallised matter, can be divided into 32 CRYSTAL CLASSES based on the morphology and internal organisation of atoms.
Crystal polyhedrons in those 32 crystal classes can be classified into six different CRYSTAL SYSTEMS. These are cubic, tetragonal, hexagonal, orthorhombic, monoclinic and triclinic crystal system.
There are 32 different combinations of elements of symmetry that define the external symmetry of a crystal, and so we can say that all mineral species, and an even larger number of all crystallised matter, can be divided into 32 CRYSTAL CLASSES based on the morphology and internal organisation of atoms.
Crystal polyhedrons in those 32 crystal classes can be classified into six different CRYSTAL SYSTEMS. These are cubic, tetragonal, hexagonal, orthorhombic, monoclinic and triclinic crystal system.
Kubični
kristalni sustav
kristalni sustav
Tetragonski
kristalni sustav
kristalni sustav
HEKSAGONSKI
KRISTALNI SUSTAV
(i TRIGONSKI podsustav)
KRISTALNI SUSTAV
(i TRIGONSKI podsustav)
Rompski
kristalni sustav
kristalni sustav
Monoklinski
kristalni sustav
kristalni sustav
Triklinski
kristalni sustav
kristalni sustav
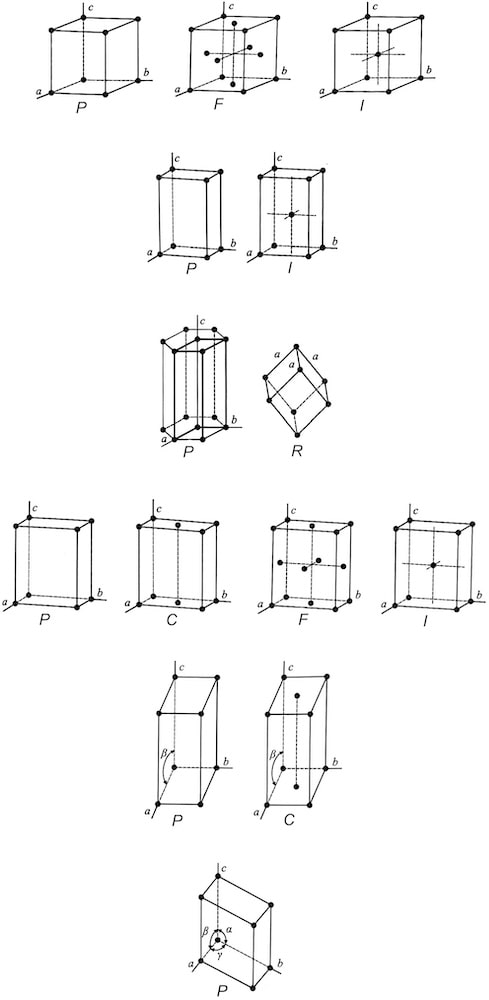
Through crystal systems, 14 different space lattices are also classified. They represent the shapes of unit cells, the basic building blocks of all minerals, which show the periodicity of repeating motifs (atoms, molecules...) in three dimensions throughout the crystal lattice - these are the so-called BRAVAIS LATTICES.
Bravais lattices – 14 different space lattices derived from the shapes of unit cells, which differ in the relationships of edges and angles between them – three cubic (P, B, F), two tetragonal (P, B), hexagonal (P), rhombohedral (P), four orthorhombic (P, B, F, E), two monoclinic (P, E), and triclinic (P).
Bravais lattices – 14 different space lattices derived from the shapes of unit cells, which differ in the relationships of edges and angles between them – three cubic (P, B, F), two tetragonal (P, B), hexagonal (P), rhombohedral (P), four orthorhombic (P, B, F, E), two monoclinic (P, E), and triclinic (P).