VII. Class of Minerals
NITRATES
Nitrates are natural salts of nitric (nitrous) acid. The main characteristics of minerals from this class are determined by their anion group, which consists of a nitrogen atom tightly bonded with three oxygen atoms. In terms of their crystal chemical features, they are very similar to carbonates as they both have a characteristic planar, triangular anion group.
Nitrates are very rare in nature, a result of their high solubility in water, and are therefore found only in exceptionally dry areas. The driest area on our planet is the Atacama Desert, which stretches along the coast of Chile, and it is here that the most famous nitrate deposits are found.
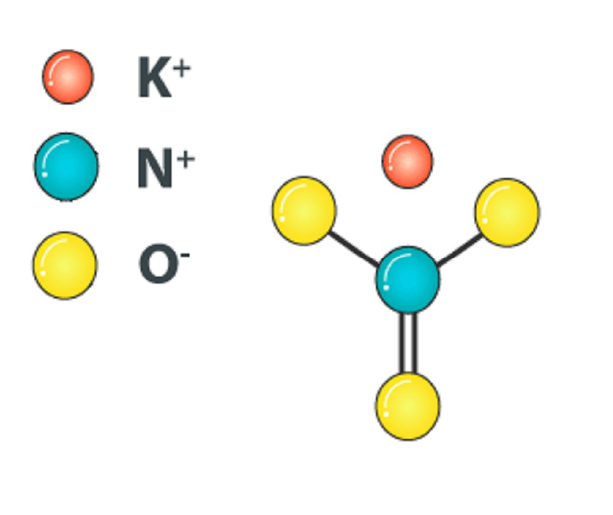
The most famous minerals from the nitrate class are saltpeter (potassium nitrate) and Chilean saltpeter (sodium nitrate), which dominate the nitrate mineral deposits in the Atacama Desert.
Chilean saltpeter is a rich source of nitrogen, so its most well-known use is in agriculture as a fertilizer, but it is also used for the production of gunpowder and other explosives. Until the beginning of the 20th century, when German chemist Fritz Haber developed a method of obtaining sodium nitrate from the atmosphere, Chilean saltpeter was the only source of sodium nitrate, which is still used today in the food industry as a preservative, in steel production, and for thermal energy storage.
Nitrates are very rare in nature, a result of their high solubility in water, and are therefore found only in exceptionally dry areas. The driest area on our planet is the Atacama Desert, which stretches along the coast of Chile, and it is here that the most famous nitrate deposits are found.
The most famous minerals from the nitrate class are saltpeter (potassium nitrate) and Chilean saltpeter (sodium nitrate), which dominate the nitrate mineral deposits in the Atacama Desert.
Chilean saltpeter is a rich source of nitrogen, so its most well-known use is in agriculture as a fertilizer, but it is also used for the production of gunpowder and other explosives. Until the beginning of the 20th century, when German chemist Fritz Haber developed a method of obtaining sodium nitrate from the atmosphere, Chilean saltpeter was the only source of sodium nitrate, which is still used today in the food industry as a preservative, in steel production, and for thermal energy storage.
NOTABLE MINERALS FROM THE NITRATE CLASS
Chilean Saltpeter
Saltpeter
Grehardite
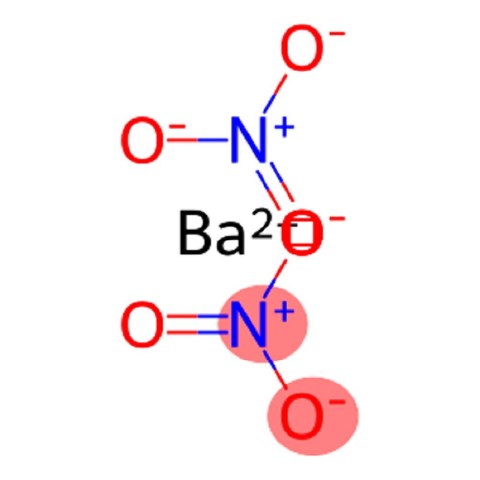
Nitrobarite